Molecular Theory of Gases
Explore the fundamental principles that govern gas behavior through molecular interactions

Introduction to Molecular Theory of Gases and Temperature
Basic concepts of molecular theory and temperature.
Learn More
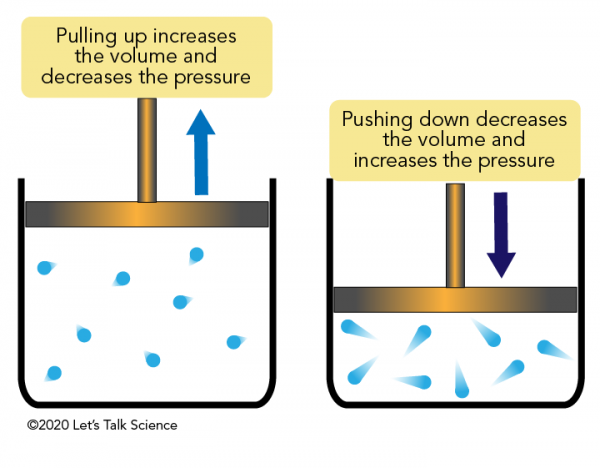
Boyle's Law
The inverse relationship between pressure and volume at constant temperature.
Learn MoreCharles' Law
The direct relationship between volume and temperature at constant pressure.
Learn MoreAvogadro's Law
The relationship between volume and the number of gas molecules at constant temperature and pressure.
Learn More
Ideal Gas Equation
The combined gas law: PV = nRT, relating pressure, volume, temperature, and moles of gas.
Learn More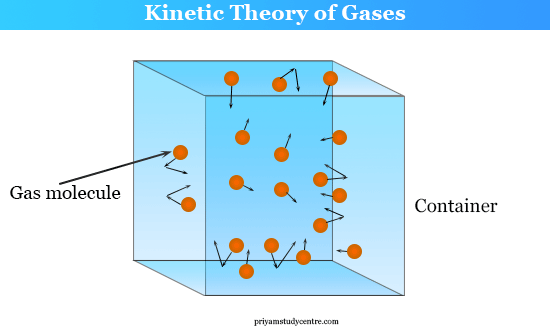
Kinetic Theory of Gases (Postulates).
The kinetic theory of gases explains the macroscopic behavior of gases (like pressure and temperature) in terms of the microscopic motion of molecules.
Learn More
Kinetic Theory of Gases (Derivation of Equation By Pressure).
The kinetic theory of gases connects the microscopic motion of molecules with the macroscopic properties of gases. Let’s derive its key results step by step.
Learn More
0 Comments